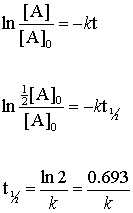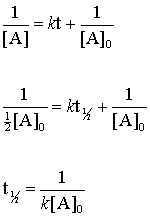half life formula for zero order reaction
T t12 and A t ½ A 0. JEE preparation requires clarity of concepts in Half Life of Zero Order Reaction.
The half-life can be defined as the time it takes for the concentration of a.

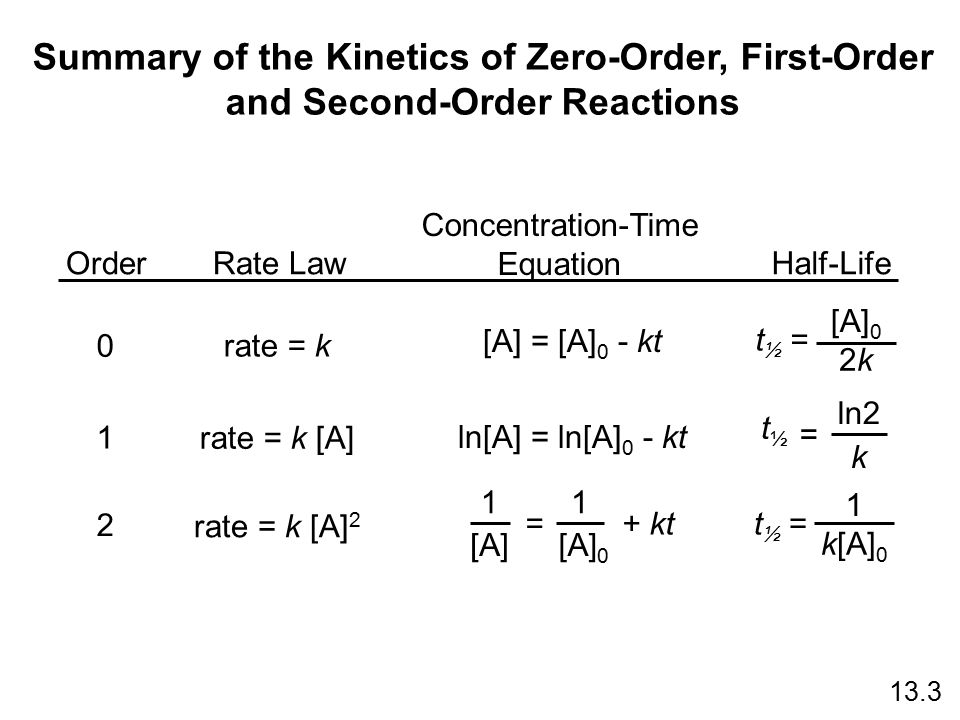
. According to the definition of half-life at time t12 the concentration of the reactant A is one-half of its initial concentration. For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao. For this discussion we will focus on reactions with a single reactant.
The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k. In some cases we need to know the initial concentration A o Substitute this information into the equation for the half life of a reaction with this order and solve for t ½. The half-life of a zero-order reaction the formula is given as t 12 R02k.
And for the second-order reaction the formula for the half-life of the reaction is given. Its application is used in chemistry and medicine to predict the concentration of a substance over time. The formula for the half-life of different reactions is given below.
The half-life period of first and zero order reaction can be calculated using the integrated rate equation- ie. Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial value. The formula for half-life in chemistry depends on the order of the reaction.
The half-life of a second-order reaction is given by the formula 1kR 0. Substituting these terms into the. The half-life of a first-order reaction is given as t 12 0693k.
The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. The concepts of half life plays a key. For a zero order reaction the formula is t½ Ao 2k.
The half-life is the time required for a quantity to fall to half its initial value as measured at the beginning of the time period. How do you find the order of a half-life reaction. The half-life of a second-order reaction is given by the formula 1kR 0.
The equation indicates that the smaller the A 0 the shorter the half-life or in other words the half-life of a zero-order reaction gets shorter as the concentration decreases. The half-life formula for various reactions is given below. For the first-order reaction the half-life is defined as t12 0693k.
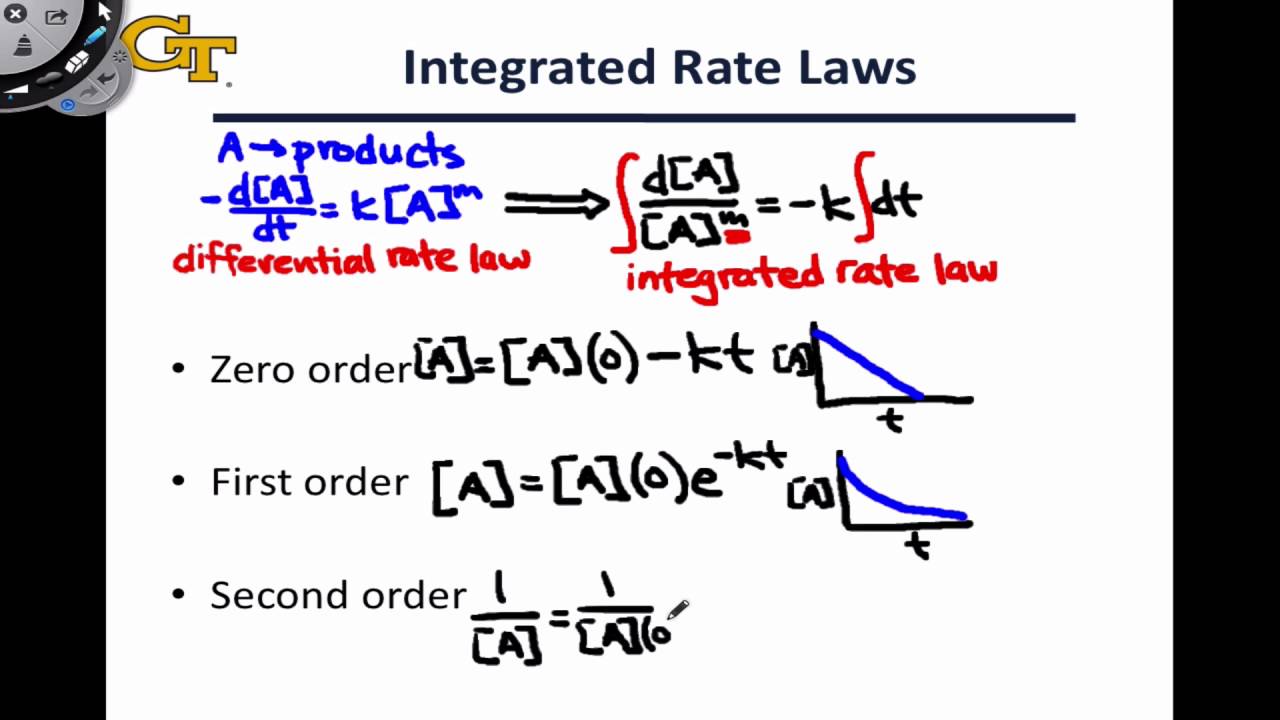
The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions. The half-life of a reaction is referred to as t 12 unit - seconds The initial reactant concentration is referred to as R 0. If we know the integrated rate laws we can determine the half-lives for first- second- and zero-order reactions.
The half-life of a first-order reaction is given as t 12 0693k. Another method for determining the order of a reaction is to examine the behavior of the half-life as the reaction progresses. Half life formula for zero order reaction The half-life of a reaction t_12 is the amount of time needed for a reactant concentration to decrease by half compared to its initial concentration.
And the reason for this is that most zero-order reactions either require a catalyst or occur between gases in saturated containers. An equation relating the half-life of a first-order reaction to its rate constant may be derived from its integrated rate law. The rate constant k for the reaction or enough information to determine it.
For zero order reaction. The order of the reaction or enough information to determine it. Click here to access solved previous year questions solved examples and important formulas based on the chapter.
The half-life equation for a zero-order reaction is t12A02k t 1 2 A 0 2 k.

Zero Order Reactions Video Kinetics Khan Academy

Half Life Of Zero Th 0th Order Reaction Derivation Youtube

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube

Calculate The Half Life Period For Zero Order Reaction 12 Chemical Kinetics Chemistry Youtube
Solved Which Of The Following Represents The Equation For A Zero Order Course Hero
13 3 Integrated Rate Laws Youtube
Derive Half Life For Zero Order And First Order Reaction Chemistry Point

Half Life Expressions Chemistnate

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube

Zero Order Reaction Definition Examples Formula

First Order Reaction Definition Example Half Life Period Chemist Notes

How To Find The Rate Constant For A Zero Order Reaction From A Graph Youtube
Summary Of The Kinetics Of Zero Order First Order Ppt Download
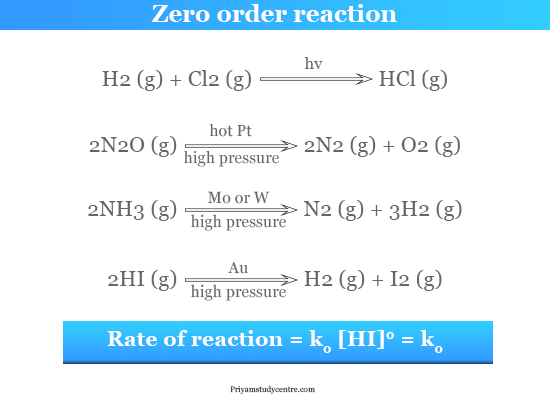
Zero Order Reaction Definition Examples Formula
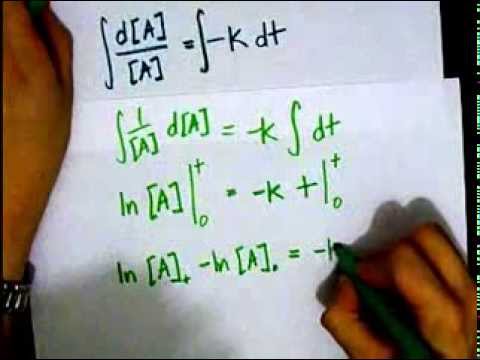
Integrated Rate Law First Order Reaction Youtube

Half Life Of A Zero Order Reaction Is 250sec T75 T100 Of The Reaction Respectively In Sec Are Edurev Neet Question